How to Calculate Partial Pressure
In the input field enter the mole fraction of the solution and the Henry constant for the mole fraction. Ivan Gutierrez discusses what conditions should be used for calculating H 2 S partial pressure when considering sour service.

Gas Mixtures And Partial Pressures Video Khan Academy
If the partial pressure of hydrogen is 1 atm find the mole fraction of oxygen in the.

. It can be calculated if you know the total. They are ideal gas law Henrys law and Daltons law. Put down for reference the equilibrium equation.
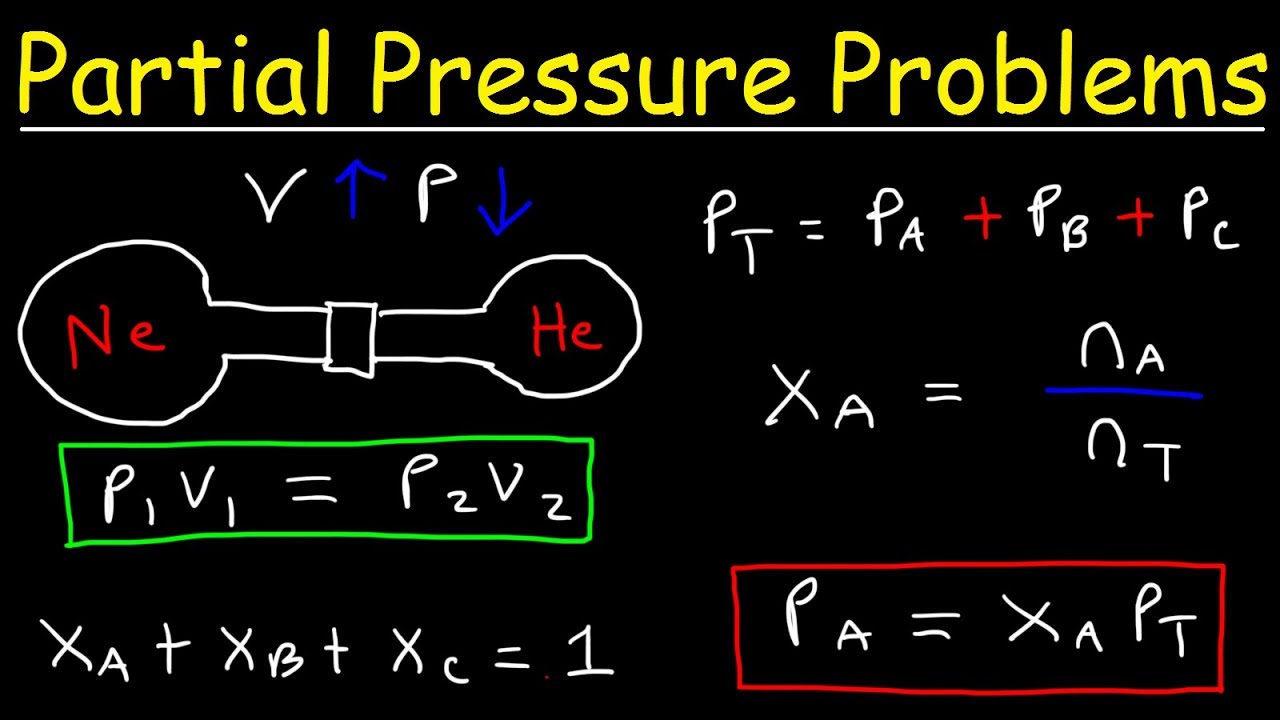
Generally partial pressure can be calculated by multiplying the mole fraction of the gas with the total pressure of container. M o l 1. Substitute those values into the.
List the equilibrium conditions in terms of x. The partial pressure of a gas can be calculated using three different laws. Answer 1 of 3.
R 008206 a t m. It corresponds to the total pressure which the single gas component would exert if it alone occupied the whole volume. Calculating Partial Pressure Calculating partial pressure of a specific gas mainly CO2 and H2S for corrosionists can be confusing.
The theory of the o2 sensor working. To calculate partial pressure start by applying the equation k PV to treat the gas as an ideal gas according to Boyles law. What is NACE MR0175 intent when it comes.
The simple formulas of each of the laws to find the partial. List the initial conditions. The partial pressure of the added CO2 must be 16 atm if the new pressure is 46atm Therefore.
The equation used is as follows we will used. An online partial pressure calculator is properly designed to calculate partial pressure volume temperature and amount of moles of each individual gas enclosed in a. Partial pressure and Kp calculations.
A mixture of hydrogen gas and oxygen gas exerts a total pressure of 15 atm on the walls of its container. Now if we divide the product of number of moles of a specific gas temperature and gas constant by volume of the entire. Then convert the equation into Kelvin if it isnt already by adding 273.
The following is the procedure how to use the partial pressure calculator. Mathematically P_Achi_A cdot P_t Where P_A. R 83145 J m o l K.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. The partial pressure of O2 and N2 remain at 20atm respectively and their total is 30atm. The partial pressure of a gas is the pressure that the gas would have if it was in the container all by itself.

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Youtube

How To Calculate Partial Pressure 14 Steps With Pictures
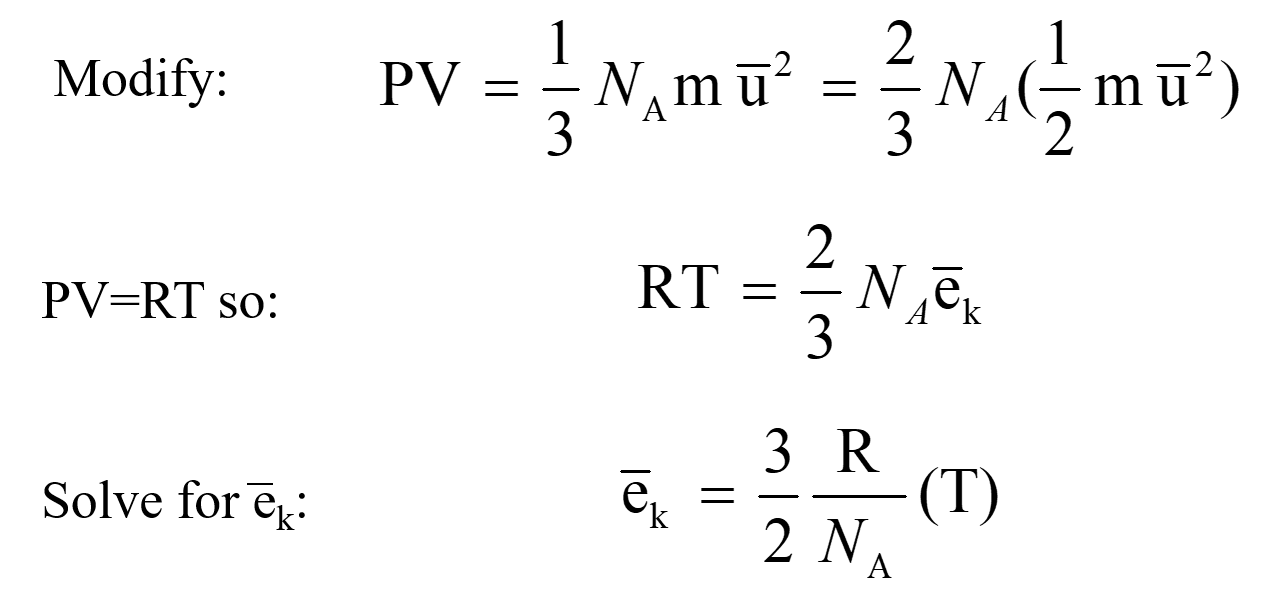
Molecular Formulas And Nomenclature
Dalton S Law Of Partial Pressure Problems Mole Fraction Chemistry Gas Laws Youtube
0 Response to "How to Calculate Partial Pressure"
Post a Comment